

Dr. Vandana Jolad Shivanagi
Founder & M.D. C PhD-UK,
B.Tech-UDCT,MBA,
EU-GMP & IRQAO-Approved Lead Auditor.
http://www.irqao.com/Search.aspx?s=jolad


Dr. Vandana Jolad Shivanagi
Founder & M.D. C PhD-UK,
B.Tech-UDCT,MBA,
EU-GMP & IRQAO-Approved Lead Auditor.
http://www.irqao.com/Search.aspx?s=jolad

Dr. Vandana, Founder and M.D. of VPC services, is herself, having strategic experience of working directly in the UK and EU for nearly 15 years in field of Complete Regulatory, cGMP, Quality, Clinical,Business Development, EU-QPPV.
Dr. Vandana is a Pharmaceutical Chemical Technologist graduated from world renowned University Department of Chemical Technology (UDCT), Mumbai (now UICT). Who further completed her PhD From University of Leeds (England) with British Overseas and Tetley Lupton Scholorship.
Dr. Vandana has overall 25 years of Pharma industry working directly on production shopfloor, QA/QC, Regulatory, and successful in one-to-one productive meetings with UK-MHRA,EU-EMEA, Other FDAs.
BUSINESS PROFILE:
Dr. Vandana Jolad Shivanagi is Consultant
of Regulatory, Pharmacovigilance, Quality, GMP, Technical Global consultant, (India). Pharma Consultancy is involved in a wide variety of regulatory consulting assignments with markets e.g. India, Europe, Asia Pacific, CIS, Middle- East, Africa, Latin America, Caribbean etc.
She is IRQAO-ASCB-UK approved LEAD AUDITOR.
Dr. Vandana assists clients by guiding the product development in line with the country
specific requirements, data generation, document compilation for submission to various Regulatory Agencies all over the world, responding to the queries and finally obtaining the approval or registration of the product all under one roof. Prime workis also GMP EU-audits.
EXPECTATIONS:
We are seeking to obtain business from Pharma manufacturers, those companies who want to get products registered in Europe and rest of the world, and we do all the back ground work of regulatory and pharmacovigilance.











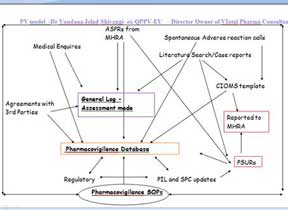


Dr. Vandana has achieved
Dr. Vandana is authorized to communicate to FDAs and Ministry of Healths, and can assist in writing for response to further information (RFI) as per client-specific requirements.